Formulating tablets with precise drug release profiles frustrates many pharmaceutical manufacturers. They struggle with inconsistent dissolution rates, compromising treatment efficacy and patient outcomes.
To control drug release rates in tablets using different HPMC binder viscosities1, manufacturers must select the appropriate viscosity grade. Higher viscosity HPMC creates thicker gel layers that slow drug release, while lower viscosity grades allow faster dissolution, enabling customized release profiles for optimal therapeutic effect.

At Kehao, we've helped numerous pharmaceutical manufacturers optimize their formulations using our premium HPMC grades. Our experience has taught us that understanding the relationship between HPMC viscosity2 and drug release kinetics is fundamental to creating effective controlled-release medications.
What is the Role of HPMC in Tablet Formulation?
Many pharmaceutical companies waste resources on failed batches due to poor excipient selection. Their tablets disintegrate too quickly or don't release medication effectively, leading to therapeutic failures.
HPMC in tablet formulation serves as a matrix-forming polymer that creates a gel barrier when hydrated, controlling drug diffusion and release. This hydrophilic polymer swells upon contact with fluids, forming a gel layer whose thickness and durability depend directly on the HPMC's viscosity grade.

HPMC's versatility in pharmaceutical manufacturing goes far beyond basic binding. As a cellulose ether derivative, it offers exceptional functionality that makes it indispensable in modern drug delivery systems. The hydroxypropyl methyl substitution pattern gives HPMC unique properties that allow formulators to design tablets with precise release profiles.
When a tablet containing HPMC contacts gastrointestinal fluids, the polymer hydrates rapidly, creating a protective gel layer around the tablet. This gel layer serves as a diffusion barrier that controls water penetration into the tablet core and regulates drug release outward. By selecting different HPMC viscosities, manufacturers can effectively engineer the thickness and persistence of this gel layer.
Lower viscosity grades (like HPMC E5 or E15) form thinner gel layers, resulting in faster drug release—ideal for products requiring prompt onset of action. Conversely, higher viscosity grades (such as HPMC K100M or K4M) create robust gel structures that significantly extend release times, perfect for once-daily dosing regimens.
Viscosity Grades and Their Applications
| HPMC Grade | Approximate Viscosity | Primary Application |
|---|---|---|
| E5 | 5 mPa·s | Immediate release |
| E15 | 15 mPa·s | Fast release |
| K4M | 4,000 mPa·s | Sustained release |
| K15M | 15,000 mPa·s | Extended release |
| K100M | 100,000 mPa·s | Very prolonged release |
Which Condition Usually Increases the Rate of Drug Dissolution from Tablets?
Pharmaceutical developers often struggle when their carefully formulated tablets dissolve too slowly. Patients experience delayed onset of action, and therapeutic failures occur when blood levels don't reach the required threshold.
Several conditions increase drug dissolution rates from tablets, with the most significant being reduced HPMC viscosity2, increased surface area through smaller particle size, higher medium temperature, addition of surfactants, and enhanced tablet porosity through disintegrants.
Understanding the interplay of factors affecting dissolution rates is crucial for pharmaceutical manufacturers seeking to optimize their formulations. In my experience working with customers across various markets, I've observed that slight modifications in formulation parameters can dramatically alter release profiles.
Temperature plays a particularly significant role in dissolution testing and real-world performance. Higher temperatures accelerate polymer chain mobility, reducing gel viscosity and increasing dissolution rates. This temperature dependence must be considered when designing formulations for global markets with varying climate conditions.
The pH of the dissolution medium also significantly impacts HPMC performance. Unlike some polymers, HPMC maintains relatively stable viscosity across a wide pH range (3-11), making it ideal for controlling drug release throughout the gastrointestinal tract. This pH-independent behavior provides consistent performance regardless of where drug release occurs in the GI tract.
Particle size of both the drug and HPMC powder affects dissolution kinetics—smaller particles provide greater surface area for hydration and dissolution. When working with poorly water-soluble drugs, micronization combined with low-viscosity HPMC grades can significantly improve bioavailability.
Dissolution Enhancement Techniques
| Technique | Mechanism | Typical Increase in Dissolution Rate |
|---|---|---|
| Lower HPMC viscosity | Thinner gel barrier | 2-5× faster |
| Micronization | Increased surface area | 3-10× faster |
| Surfactant addition | Improved wettability | 2-4× faster |
| Higher porosity | Faster medium penetration | 1.5-3× faster |
| Disintegrants | Matrix breakdown | 2-8× faster |
What Polymers Are Used for Sustained Release Tablets?
Pharmaceutical manufacturers frequently encounter stability issues with their sustained-release formulations. Their products fail dissolution testing or show inconsistent release profiles across batches, resulting in regulatory delays and market setbacks.
Common polymers used for sustained release tablets include hydroxypropyl methylcellulose (HPMC), ethylcellulose, polyethylene oxide, sodium carboxymethylcellulose, and specific methacrylate copolymers like Eudragit RS/RL, each offering unique release modification properties.
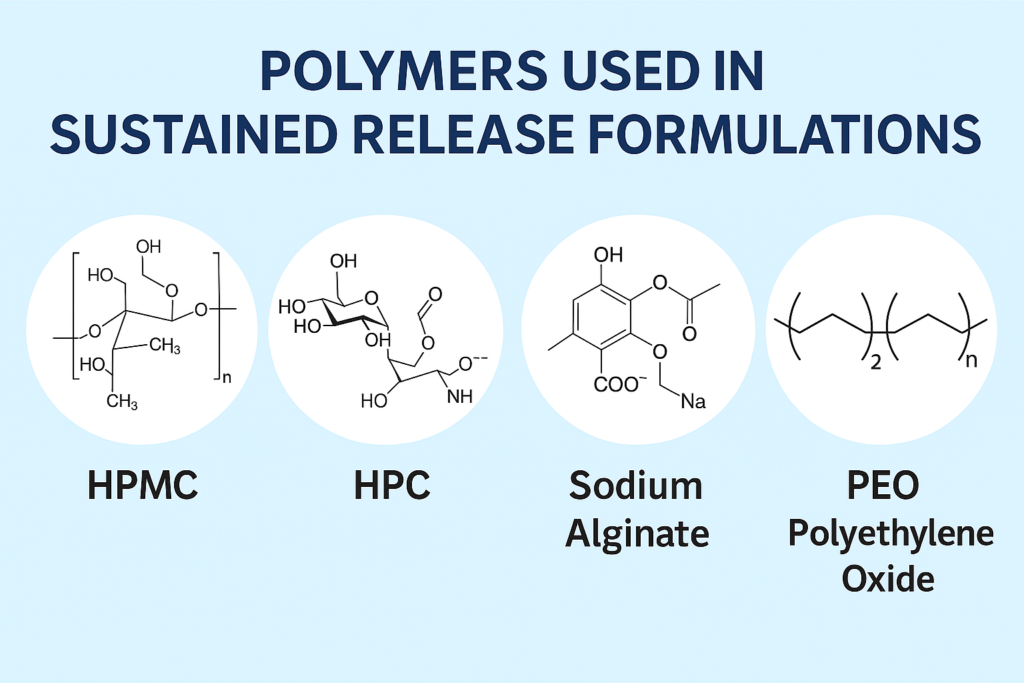
While many polymers can provide extended drug release, HPMC remains the industry gold standard due to its exceptional versatility and reliability. Our pharmaceutical customers consistently report superior performance with HPMC-based formulations compared to alternatives.
The selection of the appropriate polymer depends on numerous factors including drug solubility, desired release profile, and manufacturing considerations. For highly water-soluble drugs, higher viscosity HPMC grades or combinations with hydrophobic polymers like ethylcellulose may be necessary to achieve 24-hour release profiles.
Polymer molecular weight directly correlates with its ability to control drug release. Higher molecular weight polymers form more robust gel structures that are less prone to erosion in the gastrointestinal tract. This property explains why HPMC K100M (with approximately 100,000 mPa·s viscosity) can maintain controlled release for significantly longer periods than lower viscosity grades.
The substitution pattern of cellulose derivatives also influences performance. HPMC with higher hydroxypropyl substitution typically shows faster hydration rates but may form less robust gel structures. In contrast, higher methoxyl content tends to produce stronger gels with greater erosion resistance.
Polymer Comparison for Sustained Release Applications
| Polymer Type | Mechanism | Release Duration | pH Dependency | Manufacturing Considerations |
|---|---|---|---|---|
| HPMC | Diffusion/erosion | 4-24 hours | Low | Direct compression/wet granulation |
| Ethylcellulose | Diffusion | 8-24+ hours | Very low | Requires organic solvents |
| Polyethylene oxide | Swelling/erosion | 12-24+ hours | Low | Sensitive to oxidation |
| Carbomers | Swelling | 6-12 hours | High | pH-sensitive release |
| Methacrylate copolymers | pH-dependent dissolution | Site-specific | Very high | Requires special coating equipment |
How Does Controlled Release Medication Work?
Patients often complain about frequent dosing schedules and side effects from conventional tablets. They struggle with adherence, experiencing fluctuating drug levels that compromise treatment outcomes and quality of life.
Controlled release medications work by using specialized polymers like HPMC that form a gel matrix when hydrated, creating a diffusion barrier that precisely regulates drug release over extended periods, maintaining therapeutic drug concentrations while reducing dosing frequency.

The science behind controlled release systems3 is fascinating and complex. Unlike immediate-release formulations where drug dissolution occurs rapidly, controlled release systems employ sophisticated mechanisms to regulate the rate at which medication becomes available to the body.
In HPMC-based systems, three primary mechanisms govern drug release: diffusion, erosion, and swelling. When a matrix tablet contacts gastrointestinal fluids, water penetrates the tablet, causing the HPMC to hydrate and form a gel layer. As this happens, the tablet develops distinct regions—a dry core, a swelling region, a diffusion layer, and an erosion front.
Drug release occurs predominantly through diffusion across the gel layer, with the thickness of this layer determining release rate. Higher viscosity HPMC grades form thicker, more durable gel layers that extend the diffusion pathway, thereby slowing release. Additionally, as the outer layers of the gel matrix gradually erode, they contribute to sustained release through an erosion-controlled mechanism.
The balance between diffusion and erosion depends largely on the HPMC viscosity grade selected. With lower viscosity grades (e.g., HPMC E50), erosion plays a more dominant role. With higher viscosity grades (e.g., HPMC K100M), diffusion becomes the primary release mechanism.
Types of Controlled Release Systems
| System Type | Primary Mechanism | HPMC Viscosity Range | Typical Release Duration | Applications |
|---|---|---|---|---|
| Matrix tablets | Diffusion/erosion | 4,000-100,000 mPa·s | 8-24 hours | Chronic conditions |
| Multilayer tablets | Directional diffusion | Mixed viscosities | 12-24 hours | Pulsatile release |
| Osmotic pumps | Osmotic pressure | 5-15,000 mPa·s | 12-24+ hours | Zero-order release |
| Floating systems | Buoyancy + diffusion | 4,000-15,000 mPa·s | 6-12 hours | Gastroretentive delivery |
| Compression-coated | Delayed then controlled | Core: high; Coat: low | Time-delayed | Chronotherapeutic |
Conclusion
Selecting the appropriate HPMC viscosity grade is crucial for controlling tablet drug release rates. By understanding how viscosity affects gel formation, manufacturers can design formulations with precisely tailored dissolution profiles for optimal therapeutic outcomes.